History and Development of Electrolysis
– The word ‘electrolysis’ was coined by Michael Faraday in 1834.
– Electrolysis was used as a tool to study chemical reactions and obtain pure elements before the term was coined.
– William Nicholson and Anthony Carlisle observed the production of bubbles when wires were brought together in water, leading to the discovery of hydrogen and oxygen.
– Martin van Marum unknowingly produced electrolysis in 1785 while using an electrostatic generator.
– Luigi Galvani’s experiments with frog legs and Alessandro Volta’s subsequent tests laid the foundation for Humphry Davy’s ideas on electrolysis.
– Davy hypothesized that electrical energy is released when two elements combine to form a compound, leading to his creation of Decomposition Tables.
– Johan August Arfwedson discovered lithium in 1817, but it was William Thomas Brande who used electrolysis to isolate it in 1821.
Overview and Process of Electrolysis
– Electrolysis is the process of passing a direct electric current through an electrolyte to induce chemical reactions and decomposition.
– It requires an electrolyte, electrodes, and an external power source.
– A partition, such as an ion-exchange membrane or a salt bridge, can be used to prevent diffusion of products to the opposite electrode.
– The electrolyte contains free ions and carries electric current, either as a solution, ionic liquid, or ion-conducting polymer.
– The electrodes, made of metal, graphite, or semiconductor material, complete the electrical circuit and attract ions of opposite charge.
– Electrolysis involves the interchange of atoms and ions by the addition or removal of electrons due to the applied current.
– The desired products of electrolysis are often in a different physical state from the electrolyte and can be separated through mechanical processes.
– The quantity of products is proportional to the current, and Faraday’s laws of electrolysis describe this relationship.
– Positively charged ions (cations) move towards the cathode, while negatively charged ions (anions) move towards the anode.
– Electrons are introduced at the cathode and removed at the anode, resulting in oxidation and reduction reactions.
Industrial Uses and Manufacturing Processes
– Electrolysis is used in the Hall-Héroult process for producing aluminum.
– It is used in the electrometallurgy of various metals like lithium, sodium, potassium, and more.
– Production of chlorine and sodium hydroxide is done through the Chloralkali process.
– Electrolysis is used in the production of sodium chlorate, potassium chlorate, and perfluorinated organic compounds.
– It is also used for purifying copper, producing fuels like hydrogen, and for rust removal and cleaning of metallic objects.
– In manufacturing, electrolysis is used for electroplating and electrochemical machining.
Research Trends
– Electrolysis of carbon dioxide can produce value-added chemicals such as methane, ethylene, and ethanol.
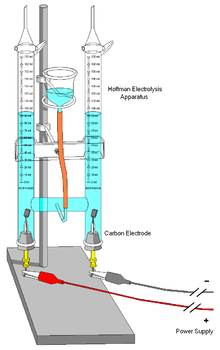
– Electrolysis of acidified water produces hydrogen and oxygen in a ratio of 2 to 1.
– Carbon/hydrocarbon assisted water electrolysis (CAWE) process utilizes carbon or hydrocarbon fuels to reduce energy input.
– Electrocrystallization is a specialized application of electrolysis used to obtain conductive crystals.
– Electrolysis of iron ore can eliminate direct emissions in steel production.
Electrolysis of Seawater
– Direct electrolysis of seawater has been investigated for hydrogen generation.
– Different types of electrolysis, including alkaline, proton-exchange membrane, and solid oxide electrolysis, have been studied.
– Proton-exchange membrane electrolysis offers a suitable combination of values in terms of investment cost, maintenance, and operation cost.
– Alkaline electrolysis is more economically feasible but has safety and environmental concerns.
– Solid oxide electrolysis requires high temperatures and suffers from degradation. Source: https://en.wikipedia.org/wiki/Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".