Types of Electrochemical Cells
– Galvanic cell (voltaic cell) generates electrical energy from spontaneous redox reactions
– Electrolytic cell drives a non-spontaneous redox reaction using applied electrical energy
– Primary cell produces current through irreversible chemical reactions and is not rechargeable
– Fuel cell generates electricity by combining fuel and oxidant through electrochemical reactions
– Concentration cell utilizes a difference in concentration of electrolytes to produce electrical energy
Galvanic Cell
– Spontaneous reaction
– Generates electric current
– Current flows through a wire, and ions flow through a salt bridge
– Anode (negative) and cathode (positive)
– Consists of two half-cells with electrodes and electrolytes
Electrolytic Cell
– Non-spontaneous reaction
– Generates current
– Current flows through a wire, and ions flow through a salt bridge
– Anode (positive) and cathode (negative)
– Decomposes chemical compounds through electrolysis
Primary Cell
– Produces current through irreversible chemical reactions
– Not rechargeable
– Used for portability, low cost, and short lifetime
– Made in standard sizes for small household appliances
– Considered wasteful and environmentally unfriendly due to high pollutant content
Fuel Cell
– A fuel cell is an electrochemical cell that converts chemical energy from hydrogen fuel and oxygen into electricity.
– Fuel cells require a continuous supply of fuel and oxygen to sustain the chemical reaction, unlike batteries.
– They are used for primary and backup power in various settings, including commercial, industrial, residential, and remote areas.
– Fuel cells can power vehicles such as forklifts, automobiles, buses, boats, motorcycles, and submarines.
– Fuel cells are classified based on the type of electrolyte they use and their startup time, ranging from seconds to minutes.
Additional Concepts:
– Secondary Cell: Lead-acid car battery is a type of secondary cell that is rechargeable and produces current through reversible chemical reactions.
– Fuel Cell Components: Anode, cathode, electrolyte, stacking of cells, and fuel source.
– Fuel Cell Efficiency: Energy efficiency, cogeneration schemes, flow batteries, and market growth.
– Related Concepts: Energy portal, activity (chemistry), cell notation, electrochemical potential, electrochemical engineering, batteries, rechargeable batteries, flow batteries, scanning flow cells.
– References: Wenzel et al. (2013), Wendt et al. (2011), Rice University (2015), Ahmad (2013), and Brett (2018).
– Environmental Impact: Manufacturing of disposable batteries requires more energy than they provide when used. Many countries are setting renewable energy goals to enter the fuel cell market. Source: https://en.wikipedia.org/wiki/Electrochemical_cell
An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells that generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.
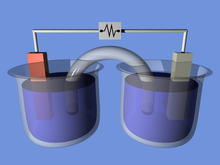
Both galvanic and electrolytic cells can be thought of as having two half-cells: consisting of separate oxidation and reduction reactions.
When one or more electrochemical cells are connected in parallel or series they make a battery. Primary cells are single use batteries.
