Electric Charge and its Properties
– Electric charge is extensive and conserved.
– Like charges repel each other, while unlike charges attract.
– An object with no net charge is electrically neutral.
– Charge is quantized and comes in integer multiples of the elementary charge.
– The elementary charge is the smallest charge that can exist freely.
Electric Charge and Subatomic Particles
– Electric charge is carried by subatomic particles.
– In ordinary matter, electrons carry negative charge and protons carry positive charge.
– If there are more electrons than protons, the matter has a negative charge.
– If there are fewer electrons than protons, the matter has a positive charge.
– If there are equal numbers of electrons and protons, the matter is neutral.
Electric Charge and Fields
– Electric charges produce electric fields.
– A moving charge also produces a magnetic field.
– The interaction of electric charges with an electromagnetic field is the source of the electromagnetic force.
– The electromagnetic force is one of the four fundamental interactions in physics.
– The study of photon-mediated interactions among charged particles is called quantum electrodynamics.
Units of Electric Charge
– The SI derived unit of electric charge is the coulomb (C).
– The elementary charge is commonly used as a unit in physics and chemistry.
– The Faraday constant represents the charge of one mole of elementary charges.
– In electrical engineering, the ampere-hour (A⋅h) is also used as a unit of charge.
– The coulomb was named after French physicist Charles-Augustin de Coulomb.
Electric Charge in Macroscopic Objects
– The electric charge of a macroscopic object is the sum of the charges of its constituent particles.
– Atoms typically have equal numbers of protons and electrons, resulting in a net neutral charge.
– Ions are atoms or groups of atoms that have lost or gained electrons, resulting in a net positive or negative charge.
– Macroscopic objects tend to be neutral overall, but can contain distributed ions or have a net charge.
– Static electricity occurs when an object has a non-zero net charge and is motionless. Source: https://en.wikipedia.org/wiki/Electric_charge
Electric charge (symbol q, sometimes Q) is the physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be positive or negative. Like charges repel each other and unlike charges attract each other. An object with no net charge is referred to as electrically neutral. Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum effects.
| Electric charge | |
|---|---|
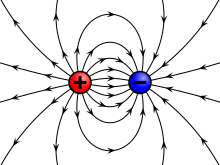 Electric field of a positive and a negative point charge | |
Common symbols | q |
| SI unit | coulomb (C) |
Other units | |
| In SI base units | C = A⋅s |
| Extensive? | yes |
| Conserved? | yes |
| Dimension | |
Electric charge is a conserved property; the net charge of an isolated system, the quantity of positive charge minus the amount of negative charge, cannot change. Electric charge is carried by subatomic particles. In ordinary matter, negative charge is carried by electrons, and positive charge is carried by the protons in the nuclei of atoms. If there are more electrons than protons in a piece of matter, it will have a negative charge, if there are fewer it will have a positive charge, and if there are equal numbers it will be neutral. Charge is quantized; it comes in integer multiples of individual small units called the elementary charge, e, about 1.602×10−19 C, which is the smallest charge that can exist freely. Particles called quarks have smaller charges, multiples of1/3e, but they are found only combined in particles that have a charge that is an integer multiple of e. In the Standard Model, charge is an absolutely conserved quantum number. The proton has a charge of +e, and the electron has a charge of −e.
Electric charges produce electric fields. A moving charge also produces a magnetic field. The interaction of electric charges with an electromagnetic field (a combination of an electric and a magnetic field) is the source of the electromagnetic (or Lorentz) force, which is one of the four fundamental interactions in physics. The study of photon-mediated interactions among charged particles is called quantum electrodynamics.
The SI derived unit of electric charge is the coulomb (C) named after French physicist Charles-Augustin de Coulomb. In electrical engineering it is also common to use the ampere-hour (A⋅h). In physics and chemistry it is common to use the elementary charge (e) as a unit. Chemistry also uses the Faraday constant, which is the charge of one mole of elementary charges.

