Types of Chemical Bonds
– Covalent Bond: Formed when two atoms share one or more pairs of electrons. Can be polar or nonpolar. Examples include water (H2O) and methane (CH4).
– Ionic Bond: Occurs when one atom donates electrons to another atom, resulting in the formation of positively charged ions (cations) and negatively charged ions (anions). Examples include sodium chloride (NaCl) and calcium carbonate (CaCO3).
– Metallic Bond: Occurs in metals when atoms share a sea of delocalized electrons. Responsible for the conductivity, malleability, and ductility of metals. Examples include copper (Cu) and iron (Fe).
– Polar Covalent Bond: Occurs when there is an unequal sharing of electrons between atoms, resulting in a partial positive and partial negative charge. Examples include water (H2O) and ammonia (NH3).
– Weak Bonds: Interactions between molecules or atoms that are weaker than primary bonds. Includes dipole-dipole interactions, London dispersion forces, and hydrogen bonding.
Bonds in Chemical Formulas
– Molecular Formulas: Indicate the chemical bonds between atoms. Can be written in different forms such as conformational, three-dimensional, or compressed two-dimensional. Non-bonding valence shell electrons and orbitals may be marked in some cases.
Strong Chemical Bonds
– Formed through the transfer or sharing of electrons between atomic centers.
– Relies on the electrostatic attraction between protons and electrons.
– Types of strong bonds differ based on the electronegativity of the elements involved.
– Electronegativity measures an atom’s tendency to attract shared electrons.
Theories of Chemical Combination
– Various theories proposed to explain how atoms attach to each other.
– Newton’s theory of particle attraction, Berzelius’ theory of electronegative and electropositive characters, Frankland, Kekulé, Couper, Butlerov, and Kolbe’s theory of valency, and Abegg’s rule.
– These theories provide insights into the nature of chemical bonds.
Atomic Models and Chemical Behavior
– Rutherford’s discovery of the atomic nucleus and electrons.
– Nagaoka’s planetary model, Bohr’s model with electron orbits, Lewis’ concept of electron-pair bonds, Kossel’s model of ionic bonding.
– Bohr’s model considers Coulomb repulsion between electrons.
– These models explain chemical behavior and the nature of chemical bonds.
Note: The content has been organized into comprehensive groups, combining identical concepts while keeping the facts, statistics, and detailed points. Source: https://en.wikipedia.org/wiki/Chemical_bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. The strength of chemical bonds varies considerably: there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding.
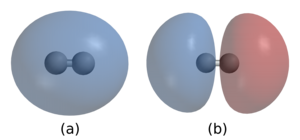
2. In (a) the two nuclei are surrounded by a cloud of two electrons in the bonding orbital that holds the molecule together. (b) shows hydrogen's antibonding orbital, which is higher in energy and is normally not occupied by any electrons.
Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. Electrons shared between two nuclei will be attracted to both of them. "Constructive quantum mechanical wavefunction interference" stabilizes the paired nuclei (see Theories of chemical bonding). Bonded nuclei maintain an optimal distance (the bond distance) balancing attractive and repulsive effects explained quantitatively by quantum theory.
The atoms in molecules, crystals, metals and other forms of matter are held together by chemical bonds, which determine the structure and properties of matter.
All bonds can be described by quantum theory, but, in practice, simplified rules and other theories allow chemists to predict the strength, directionality, and polarity of bonds. The octet rule and VSEPR theory are examples. More sophisticated theories are valence bond theory, which includes orbital hybridization and resonance, and molecular orbital theory which includes the linear combination of atomic orbitals and ligand field theory. Electrostatics are used to describe bond polarities and the effects they have on chemical substances.
