History and Structure of Cellulose
– Cellulose discovered in 1838 by Anselme Payen
– First thermoplastic polymer, celluloid, produced in 1870
– Rayon production from cellulose began in the 1890s
– Cellophane invented in 1912
– Cellulose structure determined by Hermann Staudinger in 1920
– Cellulose is hydrophilic and insoluble in water and most organic solvents
– Melts at 467°C
– Can be broken down into glucose units by treating with mineral acids
– Straight chain polymer with no coiling or branching
– Forms microfibrils with high tensile strength
Industrial Applications of Cellulose
– Used to produce paperboard and paper
– Converted into derivative products like cellophane and rayon
– Under development as a renewable fuel source
– Obtained from wood pulp and cotton for industrial use
– Used in the production of biofilms
Digestion and Nutrition
– Some animals can digest cellulose with the help of symbiotic micro-organisms
– Cellulose is a non-digestible constituent of insoluble dietary fiber
– Acts as a hydrophilic bulking agent for feces
– Potentially aids in defecation
– Cellulose content varies in different food sources
Processing of Cellulose
– Cellulose synthesized at the plasma membrane by rosette terminal complexes (RTCs)
– RTCs contain cellulose synthase enzymes
– Separate sets of CesA genes involved in primary and secondary cell wall biosynthesis
– Multiple subfamilies in the plant CesA superfamily
– UDP-glu used in cellulose synthesis by cellulose synthase enzymes
Cellulose Derivatives and Applications
– Cellulose can be dissolved in various media to produce regenerated cellulose, such as viscose and cellophane
– Cellulose esters and ethers can be formed through reactions with reagents
– Commercial applications include paper, textiles, insulation, and building materials
– Cellulose derivatives have pharmaceutical applications, such as microcrystalline cellulose in drug formulations
– Cellulose has potential as an energy crop and can be converted into butanol fuel
Note: The content has been organized into 5 comprehensive groups, combining identical concepts and including relevant facts, statistics, and detailed points. Source: https://en.wikipedia.org/wiki/Cellulose
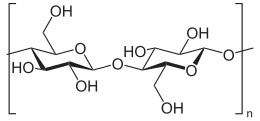
Cellulose is an organic compound with the formula (C
6H
10O
5)
n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most abundant organic polymer on Earth. The cellulose content of cotton fiber is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%.
 | |
 | |
| Identifiers | |
|---|---|
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.029.692 |
| EC Number |
|
| E number | E460 (thickeners, ...) |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| Properties | |
| (C 12H 20O 10) n | |
| Molar mass | 162.1406 g/mol per glucose unit |
| Appearance | white powder |
| Density | 1.5 g/cm3 |
| Melting point | 260–270 °C; 500–518 °F; 533–543 K Decomposes |
| none | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) | −963,000 kJ/mol[clarification needed] |
Std enthalpy of combustion (ΔcH⦵298) | −2828,000 kJ/mol[clarification needed] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp) |
REL (Recommended) | TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp) |
IDLH (Immediate danger) | N.D. |
| Related compounds | |
Related compounds | Starch |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Cellulose is mainly used to produce paperboard and paper. Smaller quantities are converted into a wide variety of derivative products such as cellophane and rayon. Conversion of cellulose from energy crops into biofuels such as cellulosic ethanol is under development as a renewable fuel source. Cellulose for industrial use is mainly obtained from wood pulp and cotton.
Some animals, particularly ruminants and termites, can digest cellulose with the help of symbiotic micro-organisms that live in their guts, such as Trichonympha. In human nutrition, cellulose is a non-digestible constituent of insoluble dietary fiber, acting as a hydrophilic bulking agent for feces and potentially aiding in defecation.

