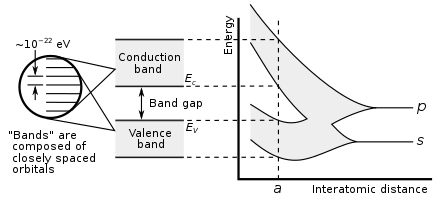
Definition and Importance of Band Gap
– Band gap is an energy range in a solid where no electronic states exist.
– It refers to the energy difference between the valence band and the conduction band.
– The band gap determines the electrical conductivity of a solid.
– Substances with large band gaps are insulators, while those with small band gaps are semiconductors.
– Conductors have either very small band gaps or none.
Band Gap in Semiconductor Physics
– Semiconductors have an intermediate-sized band gap that allows thermal excitation of electrons into the conduction band.
– Electrons can jump from the valence band to the conduction band by absorbing heat or light.
– Intrinsic semiconductors rely on thermal energy to excite charge carriers across the band gap.
– Band-gap engineering involves controlling the band gap by altering the composition of semiconductor alloys.
– The band gap of semiconductors tends to decrease with increasing temperature.
Band Gap in Insulators and Conductors
– Insulators have a larger band gap, usually greater than 4 eV.
– Insulators do not exhibit semiconductive behavior under practical conditions.
– The distinction between semiconductors and insulators is a matter of convention.
– Conductors have overlapping valence and conduction bands, resulting in no band gap.
– Electron mobility also plays a role in determining a material’s classification as a semiconductor or insulator.
Band Gap and Temperature
– The band gap energy of semiconductors decreases with increasing temperature.
– Atomic vibrations and lattice phonons affect the band gap at higher temperatures.
– Varshni’s empirical expression describes the relationship between band gap energy and temperature.
– Lattice vibrations and increased charge carriers contribute to increased conductivity at higher temperatures.
– External pressure can influence the electronic structure and optical band gaps of semiconductors.
Band Gap in Different Dimensions
– The band structure and spectroscopy vary with different dimensions: one, two, and three dimensions.
– One-dimensional non-metallic solids have optical properties dependent on electronic transitions.
– Two-dimensional structures of solids exhibit behavior due to the overlap of atomic orbitals.
– Energy splitting occurs at the Brillouin zone edge for one-dimensional situations.
– Quantum dot crystals have size-dependent band gaps and exhibit the quantum confinement effect. Source: https://en.wikipedia.org/wiki/Band_gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to the energy difference (often expressed in electronvolts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote an electron from the valence band to the conduction band. The resulting conduction-band electron (and the electron hole in the valence band) are free to move within the crystal lattice and serve as charge carriers to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there is no generated current due to no net charge carrier mobility. However, if some electrons transfer from the valence band (mostly full) to the conduction band (mostly empty), then current can flow (see carrier generation and recombination). Therefore, the band gap is a major factor determining the electrical conductivity of a solid. Substances having large band gaps (also called "wide" band gaps) are generally insulators, those with small band gaps (also called "narrow" band gaps) are semiconductor, and conductors either have very small band gaps or none, because the valence and conduction bands overlap to form a continuous band.